Before they begin to spread through the body, some cancer cells secrete molecules that may make tumor-free tissues more hospitable to invading cancer cells, CSUN Associate Professor of Biology Jonathan Kelber’s lab report in the cover article for an upcoming issue of the journal Oncotarget.
The article, lead-authored by Master’s alumna Kayla Meade alongside current Master’s student Francesca Sanchez and undergraduate researcher Analine Aguayo, reports the results of experiments on two strains of breast cancer cells, one that is metastatic — tending to spread to other parts of the body — and one that isn’t. The team grew the two cell strains in media that absorbed their secretions, then injected that “conditioned” media into mice, and tested its effects on brain and lung tissue.
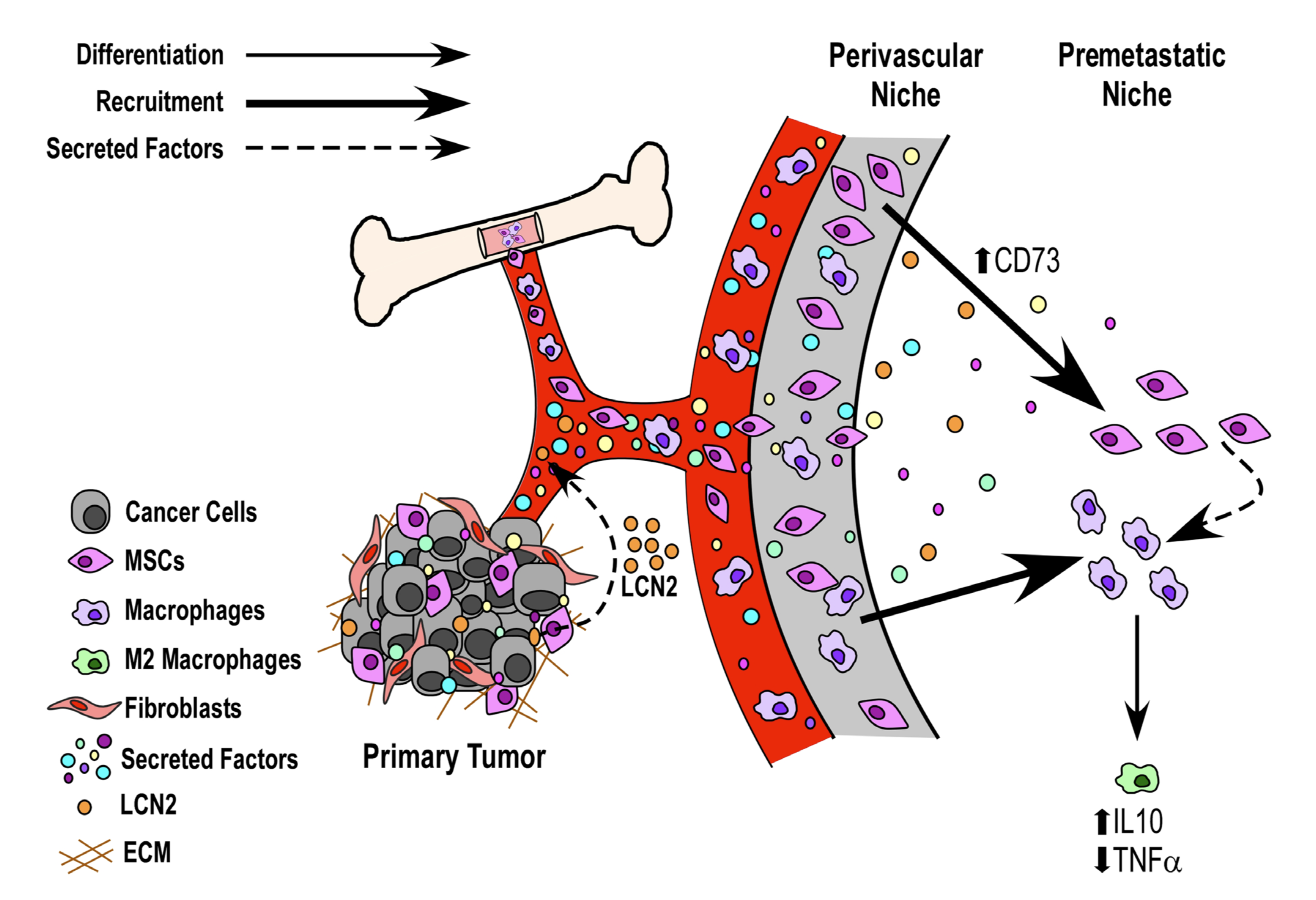
They found that, in comparison to media that hadn’t been “conditioned,” media conditioned by metastatic cancer cells altered the expression of two factors that regulate inflammation, in ways that would tend to reduce inflammatory responses. They inventoried molecules produced by the cancer cells, and constructed a map of their interactions with other genes, then searched this map for gene products that have been associated with poor outcomes in cancer patients. One of these, a gene called LCN2, is thought to have a role in the body’s response to metastatic cancer, and turned out to be strongly upregulated in the blood of the mice treated with cancer cell-conditioned media.
From these results, the authors build a model of “niche construction,” by which metastatic cancer might prompt non-cancerous tissues to create an environment that tolerates the spread of tumors. Understanding this process could open up new approaches for treating cancer, both before and after it begins to spread from its point of origin.
The full article has been posted on the journal website ahead of print publication.
Image: Figure 5 from Meade et al. (2019), the authors’ model linking cancer cell secretions to changes in host tissue gene expression that create a more tumor-tolerant environment.